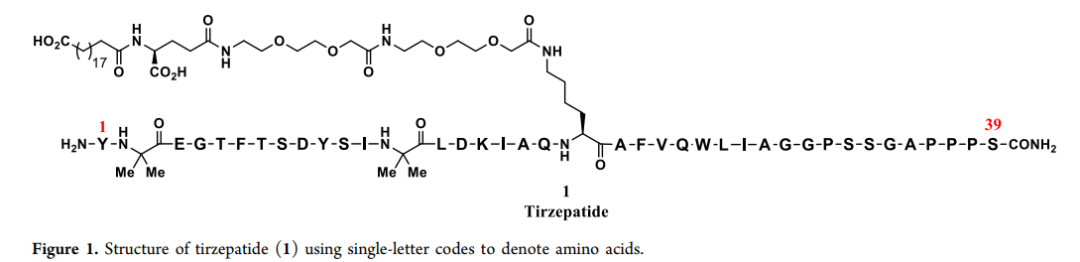
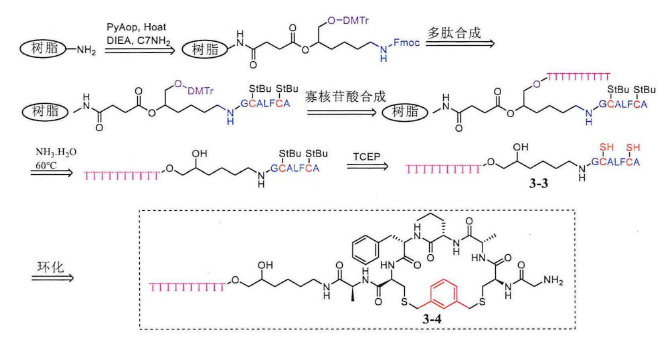
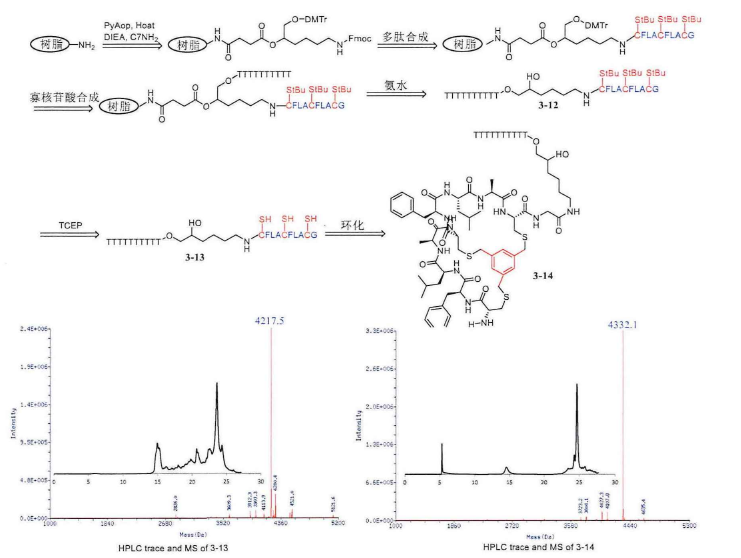
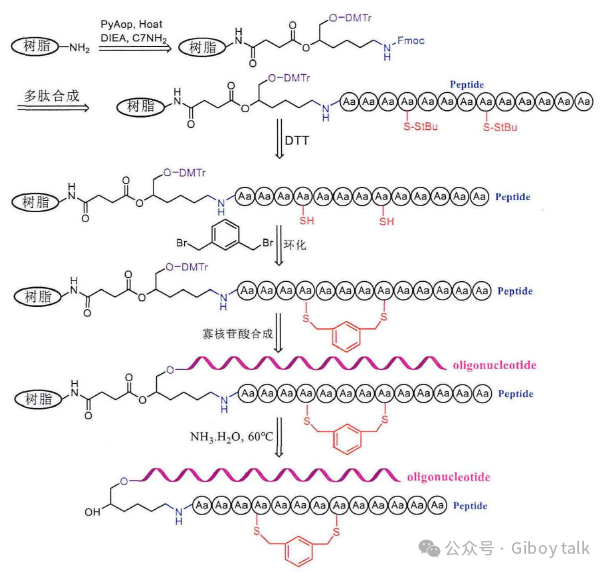
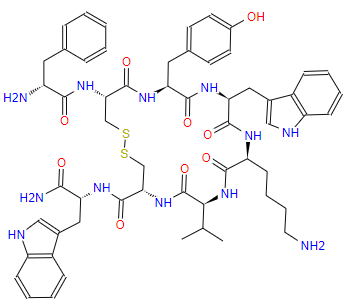
Research Progress About Amino Acid Protection In Solid-Phase Peptide Synthesis
Abstract: This paper introduces the preparation and application progress of protective amino acids ——— raw materials for peptide solid-phase synthesis reaction, discusses in detail the α-amino group, α-hydroxyl group and various new protective groups of side-chain active groups of amino acids, summarizes the structural characteristics and application conditions of new protective amino acids, and looks forward to the future development direction of protective amino acids.
Key words: Solid-phase peptide synthesis amino acid protection
In 1962, Merrifield founded the solid-phase peptide synthesis method [1], and since then, solid-phase synthesis using protective amino acids as raw materials has attracted attention for its unique advantages. In the following 10 years, the solid-phase synthesis of peptides has been continuously improved, from small-scale and short peptide chain synthesis to large-scale and long peptide chain synthesis. The anti-AIDS drug T-20, launched in 2003, is a small peptide drug composed of 36 amino acid residues that are fully synthesized. In the 90s of the last century, after the combination of combinatorial chemistry and peptide synthesis technology, the synthesis of combinatorial peptide libraries appeared, which played a huge role in promoting chemistry, biochemistry, medicine, molecular biology and other fields. In the process of development, the requirements for the protection of amino acids have also been continuously improved.
In the synthesis of solid-phase peptides, the active functional groups of the raw material amino acids participating in the reaction are protected, and the protective groups of these active groups can be removed after the reaction is completed.
When selecting amino acid protection groups, the problem of reaction selectivity should be fully considered, that is, α-amino protection groups and α-carboxyl protection groups are temporary protective groups, and an amino acid should be immediately removed after binding to the peptide chain. The side-chain protection group is a "permanent" protective group that is not affected by reactants during the synthesis of peptides, does not react, and is finally removed after peptide synthesis is completed. Therefore, the stability of the side chain protection group is greater than that of the α-amino protection group and the α-carboxyl protection group.
The selection of protective amino acids is directly related to the yield and purity of synthetic products, and the by-products and racemic formed in the reaction bring great difficulties to purification, so the development of new protective amino acids is an important part of solid-phase peptide synthesis.
At present, amino acid protection is mainly divided into three categories: α-amino protection, α-carboxyl group protection and side-chain active group protection. Based on these three classifications, this paper focuses on some new varieties of amino acid protection in recent years, as well as their structural characteristics and application conditions.
1 α-amino protection
In principle, amino groups can be reversible shielding by acylation, alkylation, alkylation and other reactions. Protective groups based on sulfur and phosphorus derivatives have also been reported. In the past 10 years, hundreds of different amino protective groups have been developed, mainly including alkoxycarbonyl protective groups, acyl protective groups and alkyl protective groups. The reported results suggest that there is no universal ideal amino protection group for peptide solid-phase synthesis.
1.1 Alkoxycarbonyl (carbamate) protective groups
This type of protective group is combined with amino acids in the form of: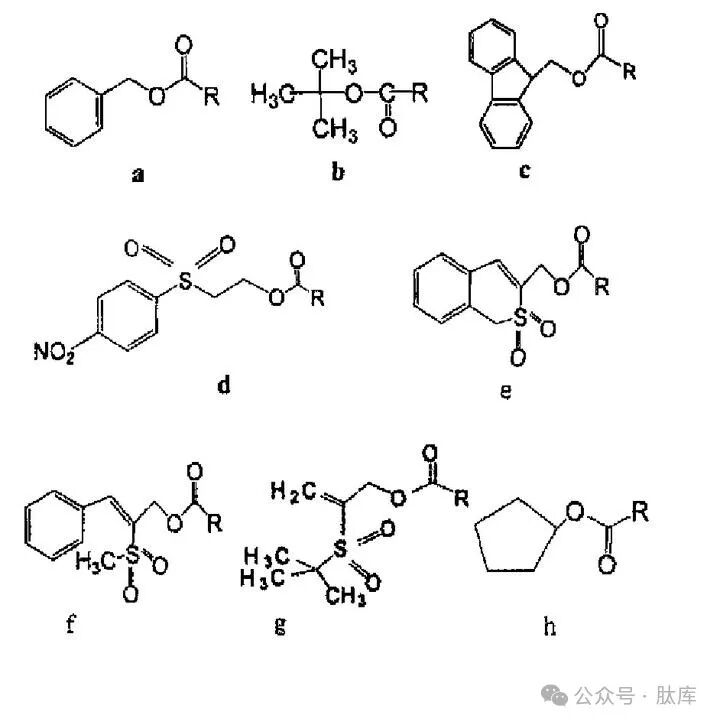
Thereinto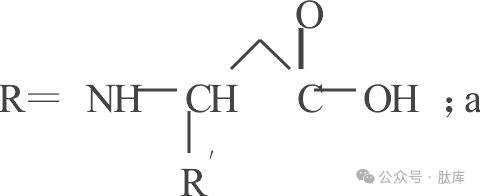
a: Benzyloxycarbonyl(Z); b: tert-butoxycarbonyl (Boc); c:9-fluoroxycarbonyl (Fmoc); d:2-(4-nitrobenzenesulfonyl)ethoxycarbonyl (NSC); e:1,1-dioxyphenyl (o)2-thiophenemethoxycarbonyl (Bsmoc); f:2-(methanesulfonyl)-3-phenyl-2-propyloxycarbonyl (MSPOC); g:2-(tert-butylsulfonyl)-2-propyloxycarbonyl (Bspoc) :h:cyclopentanoxycarbonyl (Poc)
Z, Boc, and Fmoc are the three most common amino protection groups. Z is a long-lasting amino protective group that is still widely used today. Its advantages are easy to prepare: the resulting Z-amino acids are easily crystallized and stable; It is not easy to racema during activation; The protective group can be removed under H2/Pd, HBr/AcOH, Na/liquid ammonia, etc. [2]. BOCs can be used in most peptide synthesis coupling processes and can be removed under acid hydrolysis conditions while avoiding the effects of catalytic hydrogenation, alkaline hydrolysis, and sodium/liquid ammonia reduction. Boc is usually removed under anhydrous TFA, 0°C reaction conditions [2]. FMOC is the only carbamate-type amino acid protection group that can be dissociated under weak alkali conditions, and FMOC deprotection can be done at room temperature using urine-piperidine solution or diethylamine DMF solution [3].
With the deepening of the understanding of amino protection, some amino protection groups with better selectivity or stability were obtained by modifying the functional groups on the basis of the above three protective groups.
NSCs are considered a better protective group than FMOC. NSC is more stable under alkaline conditions and less sensitive to weakly alkaline solvents. NSC is more hydrophilic than FMOC, and the possibility of asparagine formation is further reduced [4]. Consistent with FMOC, NSCs form paraffin in a deprotection reaction and eventually react with piperidine addition. However, in the Fmoc reaction, the formation of diphenylfulpenide adducts is reversible, while the adducts formed by the NSC reaction are irreversible, which reduces the probability of side reactions [5]. NSC can be removed at room temperature with piperidine, 2-aminoethanol, morpholine, liquid ammonia, etc.
Carpino et al. developed a series of novel alkali unstable amino protection groups Bsmoc, Mspoc, and Bspoct [6~8]. This type of group dissociation requires the addition of a nucleophile (such as a secondary amine) accompanied by the release of carbamates. BSMOC is stable to acids and tertiary amines, but is prone to dissociation when secondary amines are present. Since the deprotection process is also an elimination reaction, side reactions rarely occur.
Poc is a different amino protection group from Boc and Fmoc, which is stable for both acids and bases, Surajit et al. found that the Poc group is still stable after reacting with trifluoroacetic acid at 25°C for 1 h, while the side chain protection group such as tert-butyl can be completely removed, and the side chain protection group can be selectively removed by using this feature [9]. The Poc group can be removed with tetrasulfomolybdate under ultrasonic agitation, and can also be removed by HBr/AcOH or Na/liquid ammonia.
1.2 Acyl type protective groups
There are two main types of protective groups: carboxylyl and benzenesulfonyl, and the forms after combining with amino acids are as follows: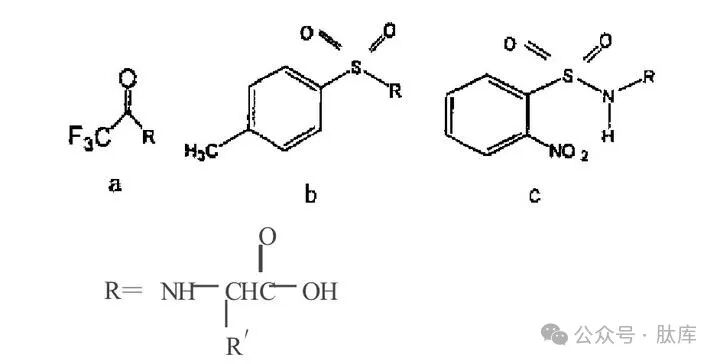
a: trifluoroacetyl group (TFA); b: p-toluene sulfonyl (Tos); c: Proximal nitrobenzene sulfonyl group (oNbs)
TFA was first introduced by Weygand in 1952 for peptide synthesis [2], and its removal conditions are relatively mild, and it can be easily removed by piperidine or sodium hydroxide. However, it is easy to racema during activation and easy to break the chain during alkaline hydrolysis, so it is widely used.
Tos is also an early protective group [3], which has very good stability and can only be removed by Na/liquid ammonia treatment, and is not affected by acid-base or catalytic hydrolysis. However, due to the troublesome operation of Na/liquid ammonia treatment and the breakage of some peptide bonds, it is rarely used at present.
The situation was greatly improved after the introduction of orthonitro to the benzene ring of Tos and the obtaining oNbs [10]. The sulfonamide protection group allows the N-acylation of amino acids without the racemic caused by the pyroxone mechanism. And this protective group can be removed by treatment with phenylthiophen or alkane mercaptan, which overcomes the shortcomings of Tfa and Tos that are not easy to remove.
1.3 Alkyl protective groups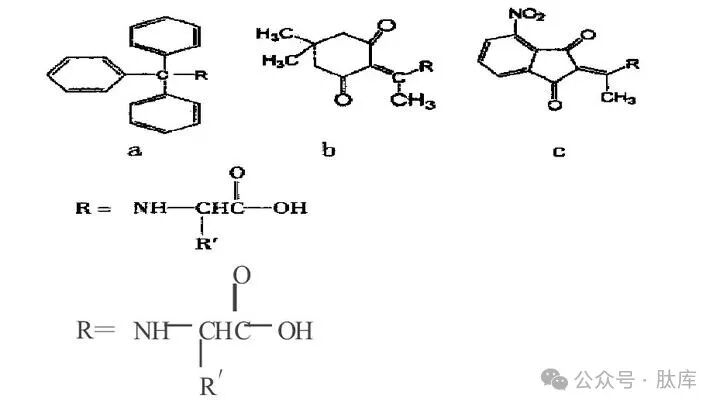
a: triphenylmethyl (Trt); b: N-1- (4, 4-dimethyl-2, 6-dioxanexyl)ethyl (Dde); c: N-1- (4-nitro-1,3-dioxinde-2-ene) ethyl (Nde)
TRT is a commonly used alkyl-type protection group [2], which can be removed with anhydrous HCI/MeOH, TFA, or HBr/AcOH under mild conditions. Because Trt has a large steric steric hindrance, it is generally difficult to introduce and bind to amino groups, and the Trt on the side chain is more stable than the Trt on the main chain, so the application of Trt is mostly used for the protection of amino acids in the side chain, while the protection of α-amino groups is less applied.
Dde and Nde are novel alkyl amino protection groups. Dde is very stable to TFA and piperidine, and it needs to be removed with 2% (v/v) hydrazine/DMF, so if the appropriate deprotection conditions are selected, the amino protection on the main chain can be retained, and the amino protection group on the side chain can be removed, and then branched-chain peptides can be synthesized.
Barrie Kellam et al. studied the characteristics of Dde, and on this basis, the resulting Nde showed good acid and secondary/tertiary base stability [11]. Similar to Dde, the structure of NDE cannot form azoledone, which effectively reduces racemic and can be removed by 2% (v/v) of hydrazine/DMF deprotection. The addition of indenium group can not only enhance the nucleophilic attack, but also improve the UV absorption value, which is conducive to online detection.
2 α-carboxyl protection
Solid-phase synthesis of peptides - generally does not require the protection of α-carboxyl groups, but only under rare conditions, it is necessary to protect α-carboxyl groups. For example, synthetic glutathione requires the γ-carboxyl group of glutamate to react, while the α-carboxyl group needs to be protected. At present, hydroxyl protection groups are roughly divided into two categories: ester groups and acylhydrazines.
2.1 Ester group
Ester protection of hydroxyl groups is the most commonly used method, mainly of the following types: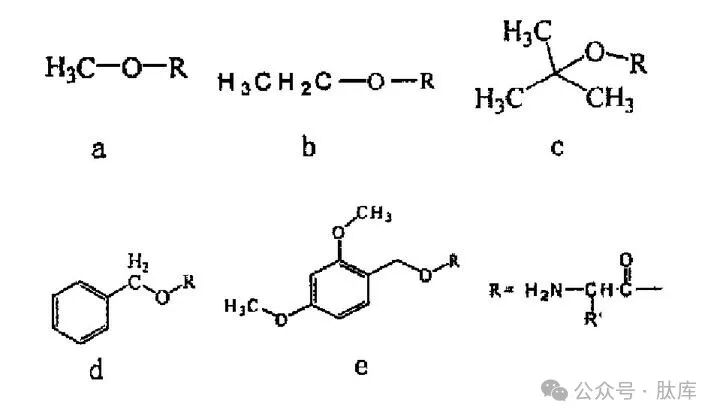
a: Me ester ; b: ethyl ester (Et); c: tert-butyl ester (tBu); d: benzyl ester (Bzl); E:2, 4-Dimethyloxyphenylmethyl ester (2, 4-DMB)
Among them, Me, Et and Bzl are prepared in simple ways and can be removed by alkali saponification. tBu is one of the most commonly used carboxyl protection groups. tBu is more stable than Me and Et, and is not easily saponified by alkali, but can be detoxified by HCI, HBr/AcOH, TFA, HF and other acids. Acid-sensitive ester 2, 4-Dmb can be removed by treatment with 1% TFA/CH2Cl2 without causing other side chain protective shedding, and this selectivity can be used in some special cases, such as the preparation of cyclic peptides by combining side chain hydroxyl groups with backbone amino groups [12].
2.2 Hydrazine
Acyl hydrazine is also used for the protection of Cα or side-chain carboxyl groups, but in this case, further azide coupling is necessary, less commonly used in solid-phase peptide synthesis. Its form is generally NH2CHRCONHNHR'.
Side chain active group protection
3.1 Ω-carboxyl protection group
Aspartic acid (Asp) and glutamic acid (G1U) have two carboxyl groups, and the omega-carboxyl groups on the side chain are prone to side reactions under the action of alkali or strong acid. For example, the lateral chain ester formed by aspartic acid during activation is generated by the corresponding intermediate state of succinimide to form isoparagine peptide. Commonly used protective groups are the tert-butyl ester group (OtBu) used in the Fmoc strategy and the β-cyclohexyl ester group (OcHex) used in the Boc strategy. These two protective groups can usually inhibit the occurrence of these side reactions, but when Asp is connected to Gly, Thr (tBu), and Cys (Acm), the side reactions are very violent and must be protected by N-2-hydroxy-4-methoxyphenyl (Hmb) [13]. When removing the protective group, TFA can be used to capture carbocations with mercaptans to reduce the occurrence of side reactions.
Karlstr⌀m and Undén synthesized two protective groups, β-2,4-dimethyl-3-amylester (ODmp) and β-3-methylpenta-3-ester group (OMpe) [14], [15]. It was found that ODmp and OMpe could effectively inhibit the asparagine conversion of aspartic acid, and could prevent the formation of asparagine more than OtBu and OcHex, which could be used for the synthesis of difficult fragments.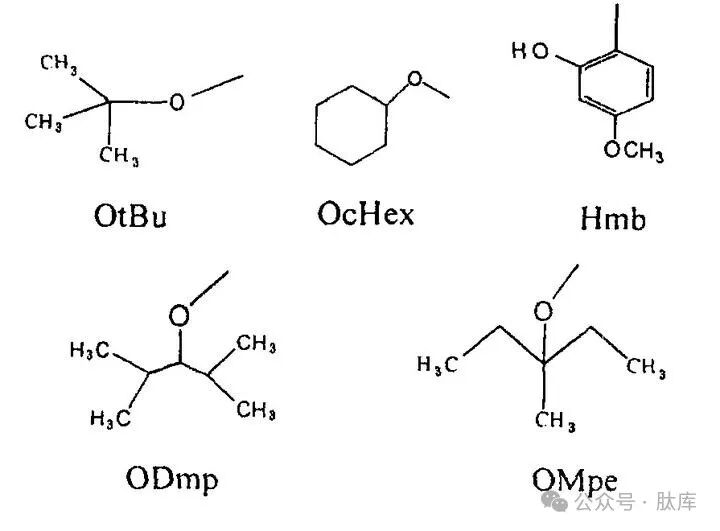
3.2 Hydroxyl protection
The phenolic hydroxyl group of tyrosine (Tyr), serine (Ser), and hydroxyl group of threonine (Thr) are all reactive groups that must be protected during the solid-phase synthesis of peptides. Commonly used groups are 2-bromophenoxycarbonyl (BrZ), benzyl (Bzl), and tert-butyl (tBu) used in the Fmoc strategy. BrZ mainly protects the phenolic hydroxyl group of Tyr, which is very stable to nucleophiles and TFA, and needs to be removed with strong acids. It is extremely unstable to bases and completely dissociated in 20% piperidine. Bzl can be removed by hydrolysis, HF, Na/liquid ammonia, and there will be partial dissociation in TFA. tBu can be removed by TFA (reaction at room temperature for 2 h), HCI/TFA or concentrated HCl at 10°C for 10 min.
Rosenthal et al. used 2,4-dimethylpentaoxycarbonyl (Doc) as the protective group of Tyr, which had a good protective effect under the Boc strategy. Doc is 1 000 times more stable than BrZ, and can be completely eliminated by HF [16].
Compared with Bzl, cHex is more stable in 50% TFA/DCM and does not form rearrangement by-products, and is also stable to 20% piperidine, so it can be used not only in Boc strategy but also in Fmoc strategy, but it is more difficult to synthesize [17].
Based on the study of all the above groups, József Bódi et al. investigated a ring-opening analogue of cHex: 3-pentanyl (Pen). It is stable not only in 50% TFA/DCM but also at 20% piperidine/DMF, so it can be used in both Boc and Fmoc strategies, and is easier to synthesize than cHex [18]. The removal of the Pen group was carried out by the HF method.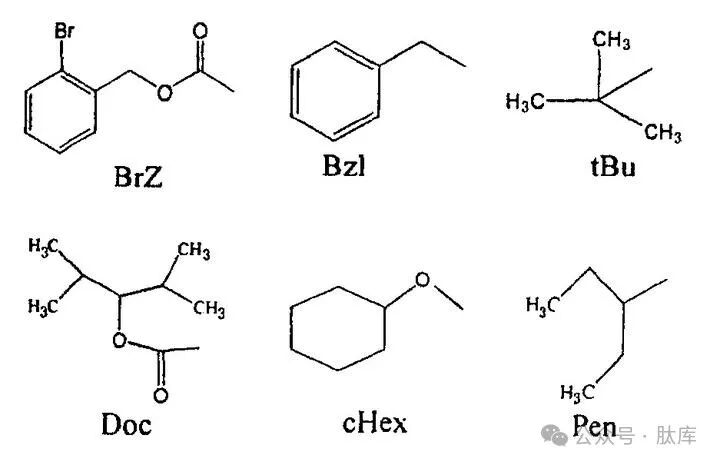
3.3 Indole protection
Tryptophan (Trp) generally does not require side-chain protection in FMOC strategies, but the tert-butylation and sulfonation of indole will intensify when other side-chain amino acid protection groups are finally removed. Boc group protection is a good way to prevent these side effects.
When the Boc strategy synthesizes Trp-containing peptides, indole is prone to cyclization or dimerization, so the indole part must be protected. Usually protected with a Nin-formic acid group (For), which is stable for treatment of repeated TFA and can be removed by alkali or HF/mercaptan. However, it also brings other problems, such as odor, polymerization side reactions of mercaptans. In addition, the Z group and 1, 3, 5-trimethylbenzene sulfonyl groups (MTS) can be used to protect Trp. However, when the Z group is removed by HF deprotection method, the reaction is incomplete. The Mts group will be gradually decomposed by TFA during peptide synthesis.
Yuji Nishiuchi et al. summarized the characteristics of Z and Mts and introduced the cyclohexyloxycarbonyl (Hoc) group. The dissociation of the carbamate-type Hoc group will form a carboxylate intermediate state, which will reduce the nucleophilic attack on the Trp indole ring [19]. In addition, the carbon ring on the Hoc group can increase the stability of bases and acids. The experimental results show that Hoc is very stable when the peptide chain is elongated and can be removed by HF without the use of thiols.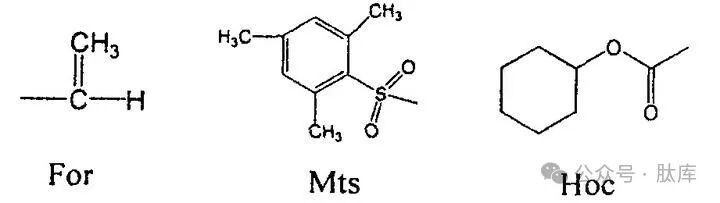
3.4 Sulfhydryl protection
The sulfhydryl groups in cysteine have the characteristics of strong nucleophilicity, easy oxidation and acidity, which require the selective shielding of sulfhydryl groups in all synthesis processes. The commonly used protective groups are Trt, tBu, and acetamide methyl group (Acm), and the deprotection conditions are different. Trt is the most widely used protective group, which can be removed by HF, TFA, I2, Hg (II.), etc.; tBu can be removed by TFMSA: Acm can be removed by I2 and Hg (II.). Modifications on the basis of the above groups yield similar tert-butyl sulfuryl (StBu) [20] and trimethylacetamide methyl (Tacm) [21], which increase the stability of these two groups and make the preparation method simpler. By taking advantage of the differences in the deprotection conditions of each protective group, the formation of disulfide bonds can be selective.
Sulfonylnitropyrimidine (NPYS) is a novel sulfhydryl protection group [22] that is unstable to the base and cannot be used in the FMOC strategy. However, if the Boc strategy is adopted, the cysteine it protects can be introduced into the N-terminus, and under weak alkaline conditions, it can be removed, so that the cyclization reaction can be carried out conveniently.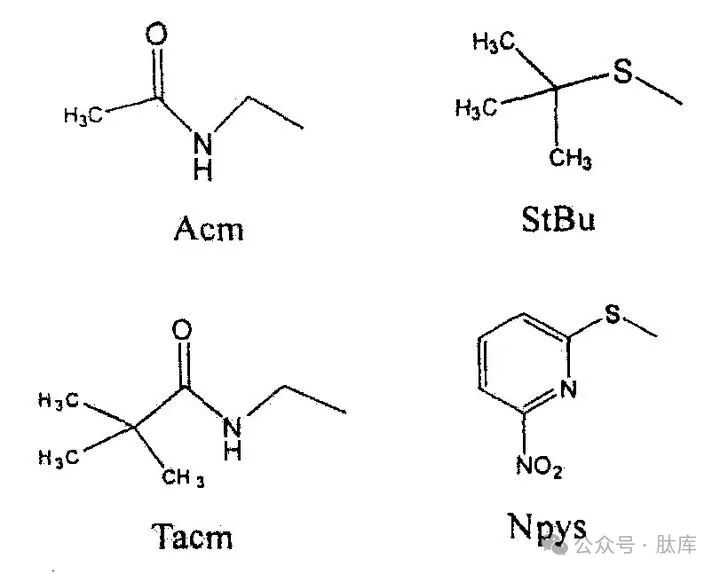
3.5 Imidazole protection
Histidine (His) is one of the most problematic amino acids in peptide synthesis. When the imidazole ring of histidine is not protected, there are two main problems: N-acylation and racemic. Reversible shielding of the azole functional group avoids these problems. The early protection groups used in the Boc strategy were Boc and Tos. The Boc group will be removed at the same time as the α-amino protection group, resulting in acylation and racemic in each step of the condensation reaction, so it is rarely used at present. Tos is the most common His protection group in the Boc strategy. It has good solubility and is easy to remove, and can be treated with HF or TFMSA. However, the Tos group is also deficient, and it will be partially removed under the action of HOBt, so when HOBt/DCC is used, side reactions such as benzosulfonylation will occur.
The benzyloxymethyl (Bom) group compensates for the above group deficiency [23], and Bom can completely shield the π-nitrogen atoms caused by racemicization by the imidazole group, and is also stable under condensation reaction conditions, and can be removed by HF or TFMSA treatment.
In the FMOC strategy, the most commonly used protective group of histidine azole is Trt. TRT shields the π-nitrogen atom of the imidazole group, which can effectively inhibit racemic. Although the reaction is relatively slow due to the large steric resistance of Trt, the steric hindrance of Trt can effectively reduce the rearrangement of alkali catalysis and reduce the occurrence of side reactions. TRT is very stable in the process of peptide synthesis, and can be easily removed with 95% TFA after synthesis.
Nπ-allyl (All) and Nπ-allyl oxymethyl (AIom) [24] are a novel class of protective groups. All and Alom act on the π-nitrogen atoms of the imidazole group, which can inhibit racemic. At the same time, it is stable to acids and bases, and can be used in Fmoc and Boc strategies, and is relatively easy to prepare, and can be removed with nucleophiles and palladium.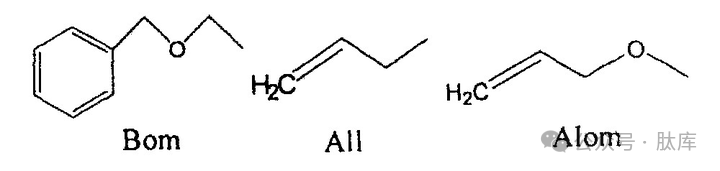
3.6 ε-Amino Protection
Lysine (Lys) is highly ε-aminobasic and nucleophilic at the same time. In the Boc strategy, it is usually protected by 2-chlorobenzooxycarbonyl [Z(2-Cl)], which has good acid stability and can prevent the phenomenon of early removal of the side-chain protection group during the process of dissociation of the Boc group. In the FMOC strategy, the Boc group is a good protective group, which has good stability to alkali and can effectively inhibit the occurrence of side reactions.
The protective groups such as Npys, Dde, and Tfa introduced earlier can also be used to protect Lys, Npys, and Tfa against piperidine instability, which are generally used in Boc strategies, and Dde must be eliminated by hydrazine detoxification, which can be used for both Fmoc and Boc strategies.
The Dde group also has its limitations, and when synthesizing long peptides, there will be partial dissociation and migration phenomena under the action of piperidine. The monomethoxytriphenylmethyl (MMT) and dimethoxytriphenylmethyl (DMT) synthesized by Sefan Matysiak et al. have enhanced stability to bases and can be used in FMOC strategies [25]. At the same time, Mint and Dmt are extremely unstable to acids, and can be removed by acetic acid/trifluoroethanol/dichloroethane (1∶2∶7, v/v) without affecting other side chain groups, so they can be used for the synthesis of cyclic peptides and branched chains.
3.7 Guanidine protection
Although the guanidine arginine group is highly basic and protected by protonation under normal reaction conditions, the corresponding activated ester has low solubility in organic solvents during activation, which is not conducive to peptide synthesis. Under some conditions, guanidine groups are also partially acylated, so protection is necessary.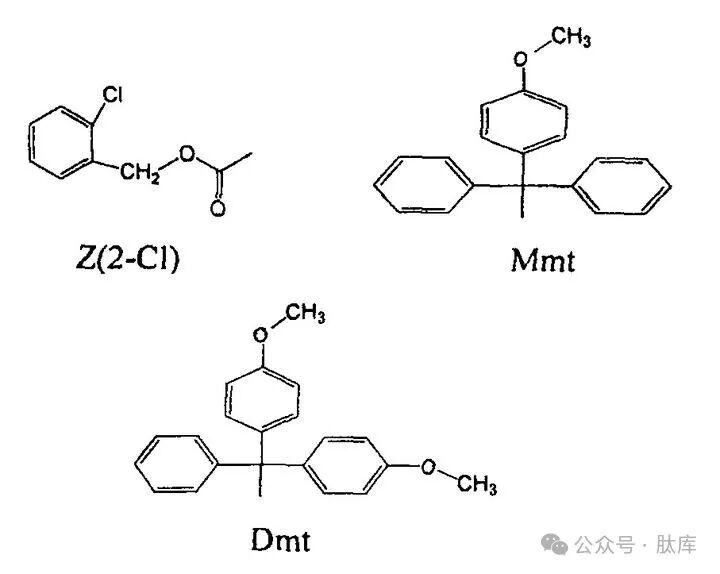
Groups such as 4-methoxybenzenesulfonyl (MBS), Mts, Tos, 4-methoxy-2, 3, 6-trimethylbenzenesulfonyl (Mtr), 2, 2, 5, 7, 8-pentamethylseman-6-sulfonyl (PMC), and 2, 2, 4, 6, 7-pentamethyl dihydrobenzofuran-5-sulfonyl (Pbf) are commonly used arginine guanidine protection groups in solid-phase synthesis [3]. The acid instability increased, in the order of Tos<Mbs< Mtr<Pmc< Pbf, and Pbf showed the best deprotection kinetics. As a temporary protective group, Mts have good compatibility with Boc, and unlike Tos, Mts can be removed by trifluoromethane sulfonic acid (TFMSA)/TFA/benzosulfide. MTR is commonly used in Fmoc strategies, but requires a longer time to deprotect with TFA/anisulfide. The acid instability of the PMC group is similar to that of the Boc group. Therefore, Boc (e.g., protective Lys) and Pmc or Pbf (protective Arg) can be optimally combined as side-chain protection groups.
3.8 Miscellaneous
3.8.1 Enzymatic hydrolysis of protective groups
When synthesizing acid-unstable or base-unstable peptide derivatives and peptide conjugates, the protective groups of amino acids need to be removed under mild or even neutral reaction conditions. Glycopeptides, phosphopeptides, and lipopeptides, as well as sulfate-containing peptides, are among these sensitive compounds. Treatment of these derivatives with alkali often results in β-elimination reactions or other adverse side reactions of Ser and Thr derivatives, while treatment with acids leads to degradation of the non-peptide fraction of the conjugate in some cases. The application of enzymatic hydrolysis of protective groups can solve the above problems.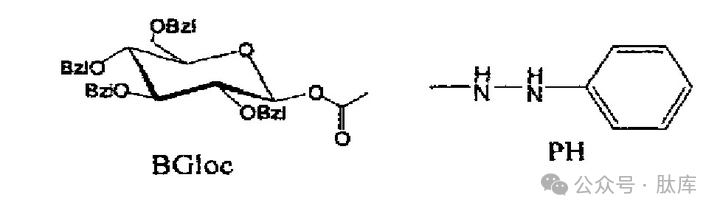
Tetraphenylglucooxycarbonyl (BGloc) is a carbamate-type enzymatic hydrolytic protection group [26], which contains O-benzyl-protected glucose as a carbohydrate moiety, which attaches carbamate oxygen atoms. After the benzoyl group is removed, the mixture of α-glucosidase from baker's yeast and β-glucosidase from almonds breaks the glucosidic bond, thereby releasing carbamate. It can be used to protect amino groups.
Phenylhydrazine (PH), studied by Gernot H.M. et al., is also an enzymatic hydrolytic protection group [27], which can be used to protect carboxyl groups, and can be used to generate acyldiazene under the action of enzymes and oxygen using tyrosinase extracted from mushrooms, and then completely removed by hydrolysis.
3.8.2 Photolytic protection groups
In 1991, Fodor introduced the photolytic protection group o-nitro-3, 4-dimethoxybenzocarbonyl (NVOC) as an amino acid protection group for the first time, but the photolysis rate of NVOC is very slow, 2-(2-nitrophenyl)propoxycarbonyl (NPPOC) is a new type of photolytic protection group, Kumar R. Bhushah et al. used it for the protection of amino groups, and found that under ultraviolet irradiation, It dissociates 2 times faster than NVOC [28].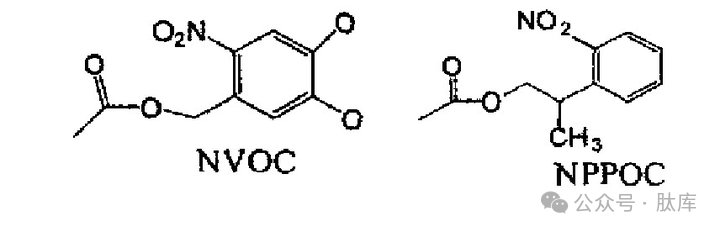
Discussion and outlook
Protective amino acids are key raw materials for peptide synthesis, and the protection strategies vary according to the different methods of synthesizing peptides.
The development of protective amino acids mainly focuses on: (1) the development of new α-amino protection groups, such as Nsc, Dde, and Nde, which have the characteristics of reducing racemic and improving the deprotection efficiency; (2) Development of protection of active groups in the side chain, a variety of protective groups with excellent stability, high selectivity and reduced occurrence of side reactions were developed for different active groups. Protective amino acids prepared in real-world applications have shown excellent performance.
With the increasing complexity of the structure of synthetic peptides, the protective groups of amino acids tend to develop in the direction of selectivity and specificity, and the enzymatic and photolytic protective groups may become new hot spots in future research due to their unique advantages.