High Yield Process Of Tirzepatide
The synthesis of tirzepatide mainly adopts the strategy of combining solid-phase synthesis (SPPS) and liquid-phase synthesis (LPPS), taking into account both efficiency and purity.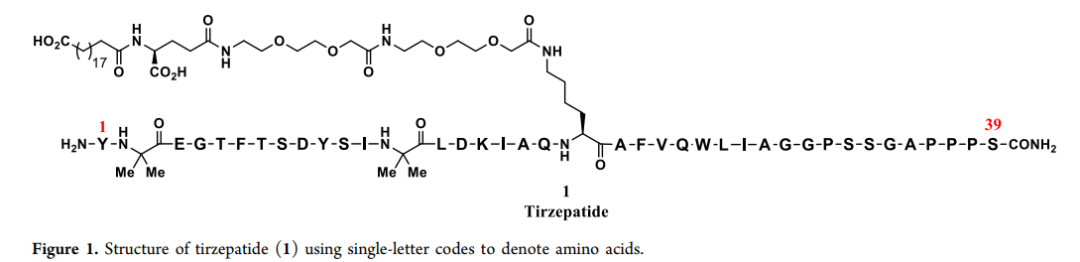
Basic Info
Name:Tirzepatide
Sequence:H-Tyr-{Aib}-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Ile-{Aib}-Leu-Asp-Lys-Ile-Ala-Gln-{diacid-C20-gamma-Glu-(AEEA)2-Lys}-Ala-Phe-Val-Gln-Trp-Leu-Ile-Ala-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH2
Molecular Formula:C225H348N48O68
Mol. wt.:4813.45
Synthesis method
The synthesis of tirpotide mainly adopts the strategy of combining solid-phase synthesis (SPPS) and liquid-phase synthesis (LPPS), taking into account efficiency and purity.
Solid-phase/liquid-phase combined synthesis method
Eli Lilly: The dual agonist of glucose-dependent insulinotropic peptide (GIP) receptor and glucagon-like peptide-1 (GLP-1) receptor developed by Eli Lilly is to synthesize multiple short peptide fragments (AA1-14, AA15-21, AA22-29 and AA30-39 fragments) by solid-phase synthesis, as shown in Figure 1 below. The final product is obtained by combining the above four fragments by liquid phase synthesis method. In the process of solid-phase synthesis of these short peptide fragments, multiple long fragments are obtained by coupling dipeptides or tetrapeptides with Fmoc or Boc protection. This reduces the amount of amino acids used while reducing the formation of impurities, improving production efficiency and product quality.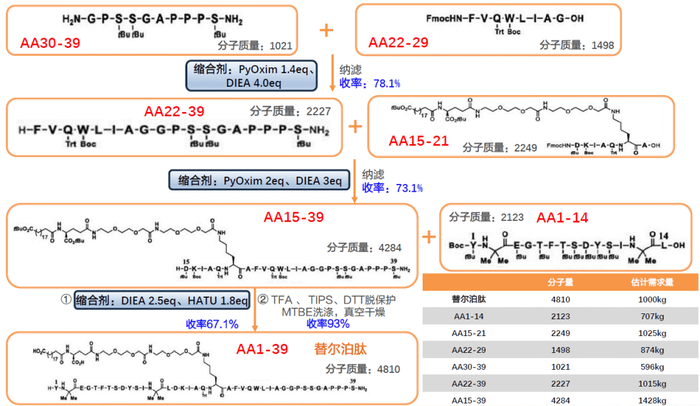
The segmented synthesis strategy of fully protected peptides was used to synthesize fully protected peptides (e.g., 1-17 and 18-39) in solid phase, and then deprotected and conjugated through liquid phase deprotection, which significantly shortened the synthesis cycle and improved the yield.
Solid-phase synthesis method
Amino acids are stepwise coupled using Rinkamide resin as a solid-phase carrier. For example, Fmoc-Ser(tBu)-OH is first fixed, then unprotected (piperidine/DMF) and then conjugated (HOBt/DIC activated carboxyl groups) until the target sequence is completed. Solid-phase synthesis method: After the synthesis, the resin is lysed with trifluoroacetic acid (TFA) and the side-chain protection group is removed to obtain crude peptides. Fragment condensation and natural chemical linkage: For long peptide chains, pre-synthesized short peptide fragments can be used for liquid phase coupling, such as by thioester bonds (e.g., NCL technology).
Key Synthesis Steps
- Amino acid activation and conjugation Use activation reagents such as HOBt, HATU, and DIC to activate carboxyl groups and condense with free amino groups to form peptide bonds. For example, complex amino acids such as Fmoc-Lys (aeea-aeea-γGlu(α-OtBu)-eicosanedioic tBu ester)-OH need to be activated step by step. Control the reaction conditions (temperature, pH, time) to reduce racemic and side reactions7.
- Protective base strategy Fmoc/tBu strategy: the amino group is protected by Fmoc, and the side chain (such as the hydroxyl group of Ser and Thr) is protected by tert-butyl (tBu)7. BOC/BZL Strategy: Applies to side-chain protection for specific amino acids.
- Lysis and crude peptide preparation The lysate is usually a DCM solution containing TFA (20% TFE), which is preliminarily purified by ether precipitation after lysis.
Purification process
Optimizing impurity control in tirpotide synthesis is a challenge and requires a combination of advanced purification technologies:
Functionalized silica gel separation column: 3-chloropropyltriethoxysilane and dimeraminocyanocyano/acetazolamide modified silica gel to enhance adsorption selectivity for target peptides and improve separation efficiency.
Combined with modified diatomaceous earth (levulinic acid treatment) to further remove hydrophobic impurities.
Chromatographic purification: Reversed-phase high-performance liquid chromatography (RP-HPLC, C18 and C8 columns) and monodisperse polymer reverse packing Seplife RP LXMS 15 (300) are used for fine purification of the end product, ensuring a purity > 98%.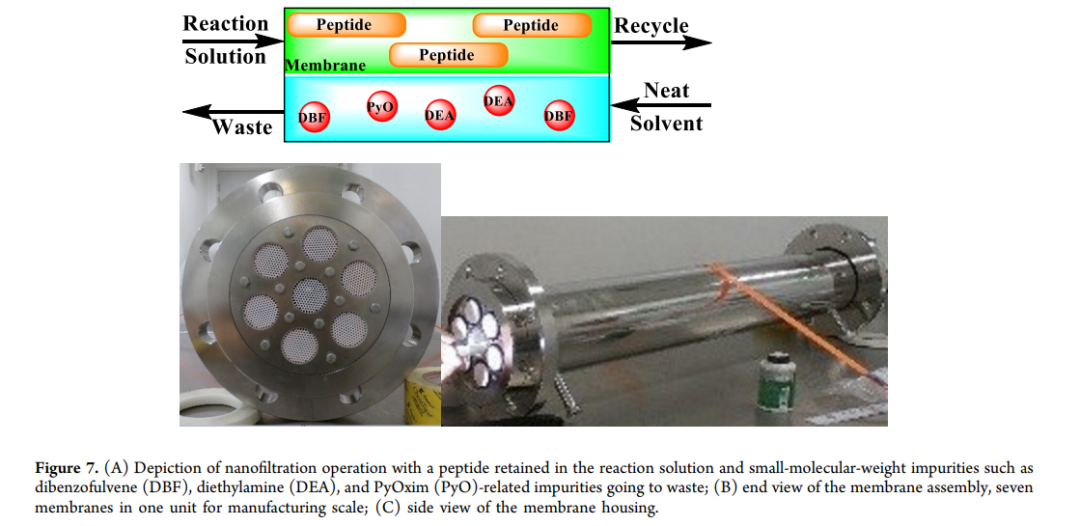
Optimization direction of synthesis process
Fragment design and connection efficiency: Optimize the fragment length (e.g., 1-17 and 18-39) and coupling conditions for solid-phase synthesis to reduce side reactions.
Biosynthesis technology: Using genetically engineered strains to express tirpotide, combined with chemical synthesis and modification, reduce costs.
Separation Technology Upgrade: Introduce nanofiltration, ultrafiltration and other technologies to simplify the post-processing process and increase production capacity.
Challenges and prospects
Impurity control: Impurities (such as missing peptides and oxidation products) in the solid-liquid mixing method are difficult to completely remove, and the purification process needs to be continuously improved. Market competition: Eli Lilly's tirpotide is 20% lower than semaglutide due to its flexible chemical synthesis path and sufficient production capacity, which puts pressure on domestic GLP-1 drug companies. Indication expansion: Tirpotide has been approved for the treatment of obstructive sleep apnea, and may be expanded to heart failure and other fields in the future, promoting further optimization of the synthesis process.
Summary
The synthesis process of tirpotide is based on the solid-liquid combination method, combined with high-efficiency activation reagents and functionalized purification technology, balancing cost and purity. In the future, through the integration of biosynthesis and chemical synthesis and the development of new separation materials, its process efficiency and product quality are expected to be further improved, supporting its continued expansion in the global diabetes and obesity treatment market.
We have been providing a wide range of solid-phase synthesis resins for various peptides in large quantities for a long time, such as 2-CTC resin, wang resin, Rink amide resin, AM(ammonia methyl resin), Sieber resin, etc. At the same time, we supply various purification fillers for peptides, recombinant proteins, antibodies, and develop purification processes.
Anyone interested, plz contact haoran.tse@gmail.com
Tel: +8618575536586
评论已关闭